immoCARE-C – the immunological test method for the specific detection of human hemoglobin in stool samples
Compared to conventional guaiac-based tests, immunological tests offer improved performance characteristics because they are not influenced by dietary factors and are more specific for detecting blood originating from the lower gastrointestinal tract. The user-friendly sample collection process increases return rates, which is particularly advantageous for colorectal cancer screening programs. The test’s detection threshold ensures an optimal balance between sensitivity and specificity, minimizing the likelihood of false results. This test serves as a valuable diagnostic tool for the early detection of colorectal cancer.
Stool sample collection is both hygienic and convenient, thanks to the sample collection tube pre-filled with buffer solution.
Patients receive the stool collection kit in an appealing folding box that includes the collection container with buffer solution and clear patient instructions.
For evaluation by medical personnel, the sample solution is applied to the test cassette. The result is read after 5 minutes by observing the appearance of purple lines in the result window.
The test’s performance characteristics are based on a single sample collection. However, the likelihood of detecting intermittent bleeding increases when multiple samples are taken. Therefore, this test is also suitable for triple sampling over three consecutive days or bowel movements, with all samples collected in one container.
Advantages of immoCARE-C:
- Results available after only 5 minutes
- Specifically detects human hemoglobin
- Simple positive/negative result interpretation
- No interference from diet
- Twice the diagnostic sensitivity compared to guaiac-based methods
The user-friendly packaging is designed for use in hospitals and clinics, with a convenient division of components: one section contains the test cassettes for laboratory analysis, while the other holds sample containers and patient instructions for easy distribution to patients.
CARE diagnostica Laborreagenzien GmbH, our highly competent partner company based in Germany, successfully uses immoCARE-C in large-scale colorectal cancer screening programs. Thanks to the proven quality and reliability of the test, immoCARE-C plays a significant role in supporting early detection of colorectal cancer.
Furthermore, the quality of the product used in these screenings—of which immoCARE-C is the central component—has been validated in several renowned studies. These studies highlight not only the test’s effectiveness but also its importance in colorectal cancer prevention and diagnostics.
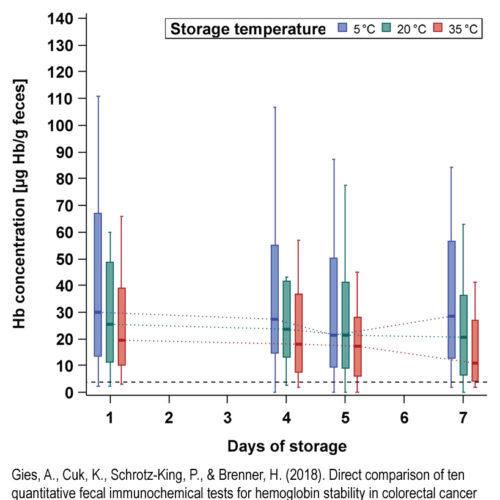
Studies conducted by the German Cancer Research Center (DKFZ) in Heidelberg have shown that the test meets the requirements set by the G-BA (Federal Joint Committee). In addition, the required sample stability at room temperature is ensured (see picture).
- Gies, A., Cuk, K., Schrotz-King, P., & Brenner, H. (2017). Direct Comparison of Diagnostic Performance of 9 Quantitative Fecal Immunochemical Tests for Colorectal Cancer Screening. Gastroenterology, 154 (1), 93–104. https://doi.org/10.1053/j.gastro.2017.09.018
- Gies, A., Gruner, L. F., Schrotz-King, P., & Brenner, H. (2019). Effect of imperfect compliance with instructions for fecal sample collection on diagnostic performance of 9 fecal immunochemical tests. Clinical Gastroenterology and Hepatology, 17 (9), 1829-1839.e4. https://doi.org/10.1016/j.cgh.2019.03.001
- Gies, A., Cuk, K., Schrotz-King, P., & Brenner, H. (2018b). Direct comparison of ten quantitative fecal immunochemical tests for hemoglobin stability in colorectal cancer screening. Clinical and Translational Gastroenterology, 9(7), e168. https://doi.org/10.1038/s41424-018-0035-2
Is the sample bottle for stool collection appropriate only for one-time or also for repeated (3x) sampling?
Several studies were carried out in the past to verify the optimal and statistically reliable number of stool samples. A study which was conducted in Germany with hemoCARE (our Guajak-Test) and 20,000 patients supported the importance of repeated sampling. We recommend triple sampling with one and the same sampling container, which means three stool samples on three consecutive days (or three consecutive defecations) collected in one sampling container to consider the possibility of bleeding on a daily basis.
Are faint lines in the result window to be evaluated as positive?
Two violet lines in the result window are visible in case of a positive result. Thereby, it must be considered that the test line may be vary in intensity. The intensity depends on the quantity of human haemoglobin in the sample. Therefore, also a very faint test lines have to be regarded as positive.
Are there any dietary requirements to be considered?
immoCARE-C is a considerably improved and more sensitive mehtod with antibodies for detecting human haemoglobin. Thus, in comparison to common methodologies, special dietary requirements do not have to be considered. Nevertheless, it is recommended - in the days before and during sampling - to consume preferably fibre-rich food to eventually stimulate bleeding-sources.
When must immoCARE-C not be used?
• during menstrual bleeding and 3 days after
• in case of diarrhoea
• in the event of bleeding hemorrhoids


